
4)社会、経済、法律及び政策の観点からの植物遺伝資源及びバイオテクノロジーの取り扱いについての学際的総合研究
A) 農業バイオテクノロジーの技術移転に関わる用件の調査
(Watanabe 2006. Elements in Technology Transfer to Developing Countries:
Cases in Agricultural Biotechnology. In : Agriculture for Peace, Taeb, M. and A. Zakri eds. United Nations University Press)
 |
過去の業績:3冊の書籍として編集。
Altman, D. W. and K. N. Watanabe 1995. Plant Biotechnology Transfer to Developing Countries. R. G. Landes Co., Georgetown, Texas, USA, 300p.
ISBN 1-57059-238-1
Watanabe, K. and E. Pehu 1997. Plant Biotechnology and Plant Genetic Resources for Sustainability and Productivity. R. G. Landes Co., Georgetown, Texas, USA, 247p. ISBN-0-12-737145-1
Watanabe, K. N. and A. Komamine 2000. Challenge of Plant and Agricultural Sciences to the Crisis of Biosphere on the Earth in the 21st Century. Landes Bioscience, Austin TX, USA. 309p. ISBN 1-58706-015-9.
B) 国際環境でのLMO (GMO) biosafetyに関わるポリシー、規制及び実施の関連事項の調査 (国連大学高等研究所との共同研究)
例:
Watanabe, K. N. 2003. Pitfalls on implementing the Cartagena Protocol on Biosafety in Japan. Nature 421 (February 13) : 689.
Watanabe, K. N., M. Taeb, and H. Okusu 2004. Japanese Controversies over Transgenic Crop Regulation. Science 305:1572.
Watanabe, K. N., M. Taeb and H. Okusu 2004.Putting Cartagena into Practice.. Nature Biotechnol. 22 (Oct).: 1207-1208.
Okusu, H. & K. N. Watanabe 2005. Regional focus on GM crop regulation. Science 308: 1409.
Kazuo N. Watanabe 1,2, Mohammad Taeb2 and Haruko Okusu2
1University of Tsukuba & 2Institute of Advanced Studies, United Nations University
An international law regulating the movement of genetically engineered organisms, Cartagena Protocol on Biosafety entered into force on September 11, 2003. However, many developing countries are unable to proceed implementing the legally binding international instrument unless they receive support in enhancing their capacity to establish an adequate regulatory environment. Our recent survey and compilation of information from regional meetings, identified as the main factors for the slow ratification and implementation process of the Protocol in many developing countries. A shift of focus from the pure theoretical and preparatory legal framework-building to the practical pilot case implementation studies is a timely and urgent requirement for enhancing the R&D and international commercialization of products derived from genetic engineering. In addition, there is also a need to harmonize the biosafety framework with various other contexts and to balance it between the trade matters and environmental concerns as well as with human health considerations.
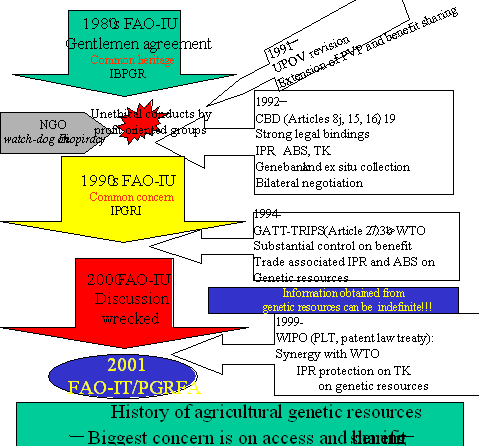
C)FAO遺伝資源条約や生物多様性条約に関わる実務上の整合性の研究
 |
D) 遺伝資源のAccess and Benefit-Sharingのケースの実施
遺伝的多様性研究にからんで実用化を目指した共同研究を海外と実施し、ここから得られる経験をABSの実例モデルとして構築しつつある。